In 2 clinical trials, Serostim® has proven efficacy in treating the 3 key symptoms of HIV‑associated wasting
Clinical Trial 1
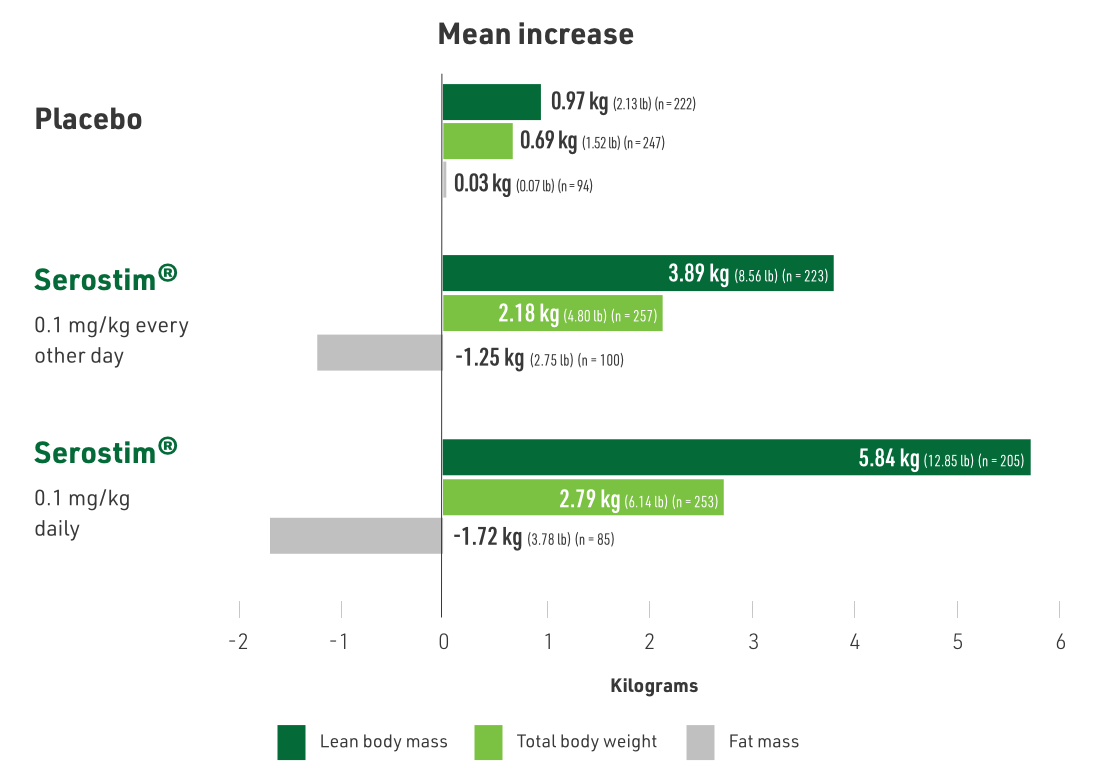
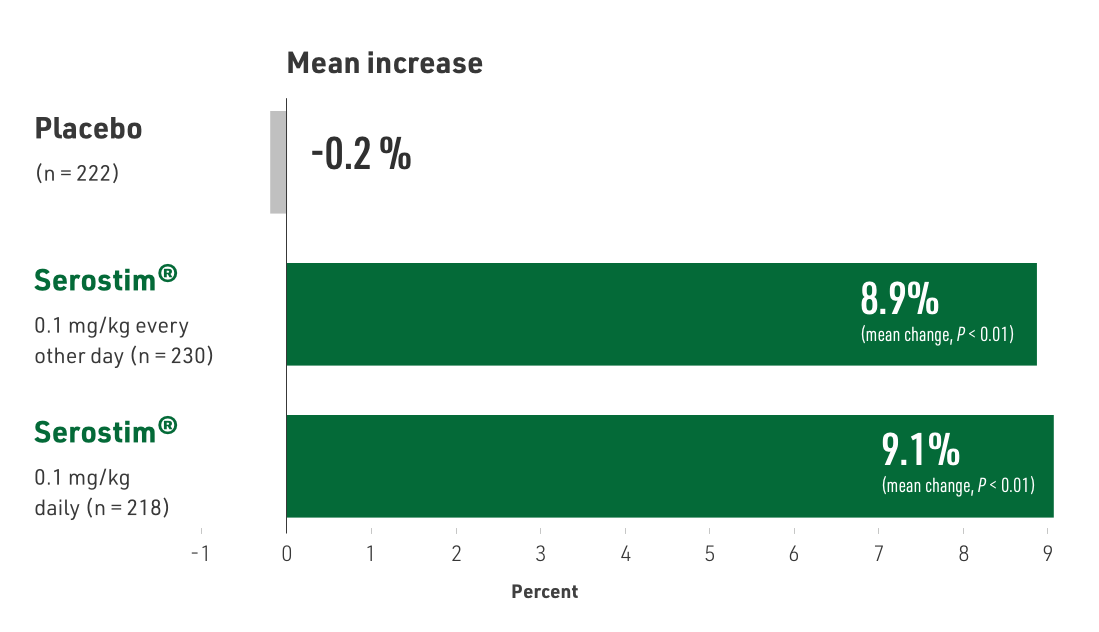
Serostim® showed statistically significant increases in LBM, body weight, and improvements in physical endurance
P < 0.001, P = 0.011, and P = 0.039, respectively
Study design
- 12-week, randomized, double-blind, placebo-controlled study followed by an open-label extension phase
- 178 patients with severe HIV‑associated wasting taking nucleoside analogue therapy (pre–highly active antiretroviral therapy [HAART] era)
- Primary endpoint: body weight (BW)
- Body composition assessed using dual energy X-ray absorptiometry (DXA) and physical function assessed by treadmill exercise testing
- Patients treated with:
- Placebo
- Serostim® 0.1 mg/kg daily
- 96% were male
- The average baseline CD4 count/microliter was 85
- 140 patients completed the 12-week course of treatment and were at least 80% compliant with the study drug